Abstract

Background: Balanced rearrangements involving the KMT2A gene (KMT2Ar) are recurrent cytogenetic abnormalities in AML, although globally they are present in only 3-7% of AML patients. Among them, the most frequent translocation is t(9;11) and it is considered of intermediate risk according to both the ELN2017 and ELN2022 classifications, while the remaining translocations involving the KMT2A gene are included in the adverse outcome group. However, there is significant controversy in the literature regarding the prognostic impact of the different KMT2Ar and some studies have only found a better prognosis in distinct subgroups of AML patients (e.g. <60 years). Moreover, the prognostic impact of gene mutations co-occurring with KMT2Ar has not been established.
Aims: To describe the mutational landscape of KMT2Ar AML and to report the potential implications of cooperating mutations in a large European cohort.
Methods: From the HARMONY Alliance AML database, a total of 205 KMT2Ar patients were selected. Estimated probabilities of overall survival (OS) were calculated using the Kaplan-Meier method and differences between survival distributions were evaluated with the log-rank test. A Cox regression model was used for multivariate OS analysis.
Results: The study population of 205 KMT2Ar AML patients included 54% females and median age was 48 years. Most of the patients (73%) had de novo AML and 43% underwent allogeneic stem cell transplantation (allo-HSCT). Median follow up was 4.9 years for those patients alive, while median OS was 1.4 years.
Translocation t(9;11) was the most frequent KMT2Ar (49%), followed by t(11;19) and t(6;11), with 16% and 12% respectively. Both t(10;11) and t(11;17) accounted for 5% of patients each, while the rest of less common translocations grouped together represented the remaining 13% of patients. OS was similar across the different translocations and we were not able to find any differences in OS between t(9;11) when compared to the remainder of the patients (p=0.755). This analysis was also carried out in a subgroup of 115 patients aged <60 years with de novo AML, with no differences in OS between t(9;11) and the rest of the patients observed (p=0.916).
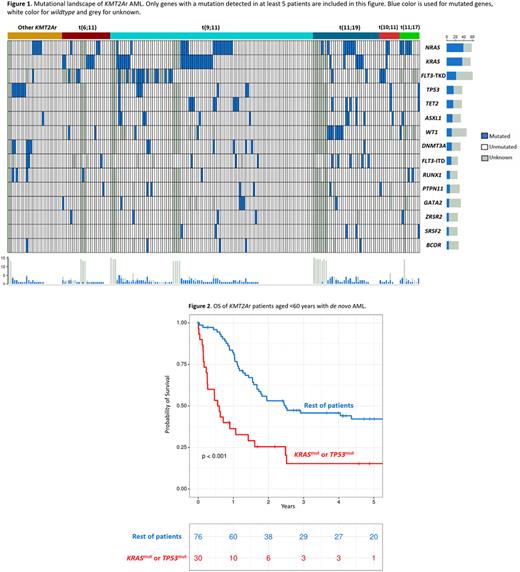
The most frequent gene mutations co-occurring with a KMT2Ar were NRAS (18.9%), KRAS (17.5%) and FLT3-TKD (10.2%), followed by TP53 (7.8%), TET2 (7.3%), ASXL1 (6.3%), DNTM3A (5.8%) and WT1 (5.8%). The distribution of mutations across different translocations involving KMT2A is shown in figure 1.
Several genes were associated with a significantly shorter OS: KRAS, TP53 and DNMT3A. Patients who presented with a mutation of any of these three genes (29% of the cohort) had a median OS of 6 months, while the rest of the patients had a median OS of 20 months (p<0.001). A multivariate Cox regression model identified the following independent variables regarding OS: secondary or therapy-related AML (HR 2.18, p=0.03), mutation of either KRAS, TP53 or DNMT3A (HR 2.15, p<0.001), age >60 years (HR 1.73, p=0.01) and allo-HSCT (HR 0.32, p<0.001). Of note, different translocations involving 11q23/KMT2A or ELN2017 categories were not significant in this model.
Mutational landscape and its prognostic impact were also explored for different subgroups. In patients aged <60 years with de novo AML, KRAS and TP53 were the most relevant genes regarding OS. Patients with a mutation of any of those two genes had a 5-year OS of 15%, while OS was 40% for the rest of the subgroup (p<0.001, figure 2). Conversely, in patients >60 years of age or in patients with secondary or therapy-related AML, DNMT3A was the most important gene with a median OS of 3 months for mutated DNMT3A and 12 months for the rest of the patients in this subgroup (p=0.041).
Conclusion/summary: Analysis of large KMT2Ar AML cohorts characterized by NGS based panel sequencing allows the discovery of concomitant gene mutations impacting patient outcome. Mutations of KRAS, TP53 or DNMT3A may identify subgroups of patients with worse OS. However, we were not able to find differences in OS between patients with t(9;11) and other translocations involving 11q23/KMT2A, both in the entire cohort and in patients <60 years with de novo AML. These findings warrant further validation, as a better understanding of gene-gene interactions has the potential to further improve patient management in KMT2Ar AML.
Disclosures
Sobas:Celgene/BMS: Honoraria; Novartis: Honoraria. Metzeler:Pfizer: Consultancy; Novartis: Consultancy; Jazz Pharmaceuticals: Consultancy; Astellas: Honoraria; Daiichi Sankyo: Honoraria; Celgene/BMS: Consultancy, Honoraria, Research Funding; AbbVie: Honoraria. Pratcorona:Novartis: Honoraria. Thiede:Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen Pharmaceuticals: Speakers Bureau; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; AgenDix GmbH: Current Employment, Current equity holder in private company; Kronos Bio, Inc.: Honoraria. Sanz:La Hoffman Roche Ltd.: Other: Advisor or review panel participant; Novartis Oncology: Consultancy; Celgene Corporation: Consultancy; Janssen Pharmaceuticals, Inc.: Other: Teaching and Speaking; takeda: Honoraria; Abbvie Pharmaceuticals: Other: Advisor or review panel participant; Helsinn: Honoraria, Other: Advisor or review panel participant; Takeda Pharmaceuticals Ltd: Other: Advisor or review panel participant. Döhner:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Research Funding; Agios: Research Funding; BMS/Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Jazz: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Kronos: Research Funding. Heuser:Kura Oncology: Consultancy; Glycostem: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; BMS: Consultancy; Agios: Consultancy, Research Funding; Takeda: Honoraria; Novartis: Consultancy, Honoraria, Research Funding; Janssen: Honoraria; Jazz Pharmaceuticals: Consultancy, Honoraria, Research Funding; Eurocept: Honoraria; Abbvie: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Research Funding; PinotBio: Consultancy, Research Funding; Roche: Consultancy, Research Funding; Tolremo: Consultancy; Astellas: Research Funding; Bayer Pharma AG: Research Funding; BergenBio: Research Funding; Loxo Oncology: Research Funding. Haferlach:Munich Leukemia Laboratory: Current Employment, Other: Part ownership. Turki:CSL Behring: Consultancy; MSD: Speakers Bureau; Jazz Pharma: Speakers Bureau. Reinhardt:BMS: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Cerus: Membership on an entity's Board of Directors or advisory committees; Medac: Membership on an entity's Board of Directors or advisory committees; EUSA Pharma: Membership on an entity's Board of Directors or advisory committees; BlueBird Bio: Research Funding. Schulze-Rath:Bayer Pharma AG: Current Employment, Current equity holder in private company. Hernández-Rivas:BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Research Support, Speakers Bureau; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy, Honoraria; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Research support, Speakers Bureau; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Research Support; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AstraZeneca: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Beigene: Membership on an entity's Board of Directors or advisory committees; Lilly: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Rovi: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees. Ossenkoppele:Gilead: Consultancy, Honoraria; Astellas: Consultancy, Honoraria; AGIOS: Consultancy, Honoraria; Servier: Consultancy, Honoraria; JAZZ: Consultancy, Honoraria; AMGEN: Consultancy, Honoraria; BMS/Celgene: Consultancy, Honoraria; Abbvie,: Consultancy, Honoraria; Novartis: Consultancy, Honoraria. Döhner:Syndax Pharmaceuticals Inc.: Consultancy, Honoraria; Janssen Pharmaceuticals: Consultancy, Honoraria; Servier: Consultancy, Honoraria; Brystol Myers Squibb: Consultancy, Honoraria, Research Funding; Astellas Pharma Inc.: Consultancy, Honoraria, Research Funding; AstraZeneca: Honoraria; Berlin-Chemie AG: Consultancy, Honoraria; Kronos Bio, Inc.: Research Funding; Pfizer Inc.: Research Funding; Amgen Inc.: Consultancy, Honoraria, Research Funding; Agios Pharmaceuticals: Consultancy, Honoraria, Research Funding; AbbVie Inc.: Consultancy, Honoraria, Research Funding; Daiichi Sankyo Co, LTD: Consultancy, Honoraria; Gilead Sciences, Inc.: Consultancy, Honoraria; Jazz Pharmaceuticals: Consultancy, Honoraria, Research Funding; Novartis AG: Consultancy, Honoraria, Research Funding. Bullinger:Astellas: Honoraria; Celgene/BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bayer Oncology: Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal